Kasetsart University, Department of Chemistry, Bangkok, Thailand
Development of efficient electrocatalysts toward oxygen reduction reaction for proton exchange membrane fuel cells (PEMFCs)
Fuel cells (FCs) are in the limelight of the energy conversion field because of their high-power density and eco-friendly nature. Nevertheless, the cosmically commercialization is still restricted by the high cost and instability of platinum (Pt)-based catalysts. Regarding these facts, it is highly desirable to exploit efficient platinum alternative catalysts for FCs, particularly for the sluggish oxygen reduction reaction (ORR) on the cathodes.
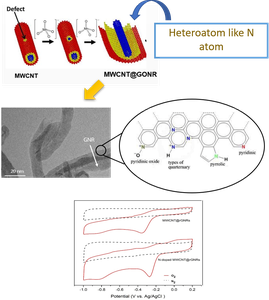
Research in our laboratory focuses on designing improved activity ORR catalysts, with significantly reduced or eliminate platinum contents. Graphene nanoribbons (GNRs), as novel carbonic material, have attracted intensive attention due to their superior physical and chemical properties. Although the surface integration endows GNRs with excellent physical properties such as electrical conductivity and thermal transmission, the chemically stable sp2 hybridized carbon atoms in GNR framework hinder their further applications in chemistry, particularly in catalysis. Up to now, many strategies have been employed to tailor or modify the electronic properties of graphene via chemical or physical methods. Both theoretical calculations and experiments have proved that chemical doping with heteroatoms like N can intrinsically modify the properties of carbon materials.
Our aim is to develop an improved understanding of the ORR process occurring on various surfaces, providing fundamental insight that can rationally guide our design of new catalyst materials.